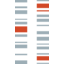

Intellia Therapeutics, Inc., a genome editing company, focuses on the development of therapeutics. The company's in vivo programs include NTLA-2001, which is in Phase 1 clinical trial for the treatment of transthyretin amyloidosis; and NTLA-2002 for the treatment of hereditary angioedema, as well as other liver-focused programs comprising hemophilia A and hemophilia B, hyperoxaluria Type 1, and alpha-1 antitrypsin deficiency. Its ex vivo pipeline includes NTLA-5001 for the treatment of acute myeloid leukemia; and proprietary programs focused on developing engineered cell therapies to treat various oncological and autoimmune disorders. In addition, it offers tools comprising of Clustered, Regularly Interspaced Short Palindromic Repeats/CRISPR associated 9 (CRISPR/Cas9) system. Intellia Therapeutics, Inc. has license and collaboration agreements with Novartis Institutes for BioMedical Research, Inc. to engineer hematopoietic stem cells for the treatment of sickle cell disease; Regeneron Pharmaceuticals, Inc. to co-develop potential products for the treatment of hemophilia A and hemophilia B; Ospedale San Raffaele; and a strategic collaboration with SparingVision SAS to develop novel genomic medicines utilizing CRISPR/Cas9 technology for the treatment of ocular diseases. The company was formerly known as AZRN, Inc. Intellia Therapeutics, Inc. was incorporated in 2014 and is headquartered in Cambridge, Massachusetts.
P/B ratio for Intellia Therapeutics, Inc. (NTLA)
P/B ratio as of 2023: 2.58
According to Intellia Therapeutics, Inc.'s latest financial reports and stock price the company's current price-to-sales ratio (TTM) is 2.58. At the end of 2022 the company had a P/B ratio of 2.17.
P/B ratio history for Intellia Therapeutics, Inc. from 2014 to 2023
P/B ratio at the end of each year
| Year | P/B ratio |
|---|---|
| 2023 | 2.58 |
| 2022 | 2.17 |
| 2021 | 8.06 |
| 2020 | 5.78 |
| 2019 | 2.57 |
| 2018 | 2.12 |
| 2017 | 2.30 |
| 2016 | 1.39 |
| 2015 | 5.80 |
| 2014 | 1.42 |

